
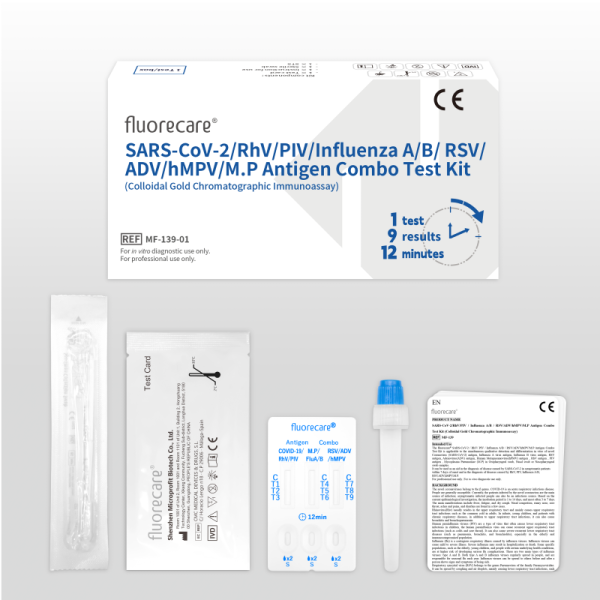








fluorecare SARS-CoV-2/RhV/PIV/Influenza A/B / RSV/ADV/hMPV/M.P Antigen Combo Test Kit (Colloidal Gold Chromatographic Immunoassay) MF-139
fluorecare SARS-CoV-2/RhV/PIV/Influenza A/B / RSV/ADV/hMPV/M.P Antigen Combo Test Kit (Colloidal Gold Chromatographic Immunoassay) MF-139
- Model: MF-139
- Brand: fluorecare
- Specs:
We analyzed papers on respiratory infections published in Nature Communications and EUROPEAN RESPIRATORY review and found that Influenza A virus, influenza B virus, respiratory syncytial virus (RSV), rhinovirus(RhV), adenovirus(ADV) and parainfluenza virus(PIV) are the most frequent respiratory viruses that cause acute respiratory viral infections.
The research also point out based on the proportion of positive detection for bacteria,Streptococcus pneumoniae (S.p) was the most frequent bacterium, accounting for 29.9% of the total positive detection,followed by Mycoplasma pneumoniae (M.p,18.6%),Haemophilus influenzae (H. influenzae, 15.8%), Klebsiella pneu moniae (K. pn, 12.5%), Pseudomonas aeruginosa (P.aeruginosa, 11.4%), Staphylococcus aureus (S. aureus, 8.9%),Chlamydia pneumoniae (C. pneumoniae, 1.6%), Legionella pneumophila (L. p, 0.9%) and Group A Streptococcus(GAS, 0.4%) .
Therefore, we developed SARS-CoV-2/RhV/PIV/Influenza A/B / RSV/ADV/hMPV/M.P Antigen Combo Test Kit , which can test about 95.3% frequent respiratory pathogens rapidly to facilitate guidance of medication.

√1 Sample
√9 Results
√Results in 12 mins
√CE Certification
√High Sensitivity & Specificity
| Virus Strain | LoD | Sensitivity | Specificity |
SARS-CoV-2 (COVID-19) | SARS-CoV-2 | 1.8×105 TCID50/mL | 92.93% | 100.0% |
Rhinovirus(RhV) | Rhv | 4.8mg/mL | 97.48% | 98.68% |
Parainfluenza viruses(PIV) | PIV 1 | 4.6mg/mL | 98.08% | 98.75% |
PIV 2 | 3.3mg/mL | |||
PIV 3 | 4.1mg/mL | |||
Mycoplasma Pneumoniae(M.P) | Mycoplasma Pneumoniae | 8.12×106 CFU/mL | 93.93% | 96.91% |
Influenza A (FLU A) | 2009H1N1 | 9.8×106 TCID50/mL | 96.71% | 94.98% |
Seasonal H1N1 | 1.3×107 TCID50/mL | |||
Type A H3N2 | 2.1×108 TCID50/mL | |||
Influenza B (FLU B) | B/Victoria | 1×106 TCID50/mL | 95.22% | 93.64% |
B/Yamagata | 1×106 TCID50/mL | |||
Respiratory syncytial virus (RSV) | RSV type A | 4.6×108 TCID50/mL | 95.41% | 95.17% |
RSV type B | 3.2×107 TCID50/mL | |||
Adenovirus (ADV) | Adenovirus 3 | 20μg/mL | 94.16% | 94.04% |
Adenovirus 7 | 2μg/mL | |||
Adenovirus 40 | 10μg/mL | |||
Adenovirus 41 | 15μg/mL | |||
Adenovirus 55 | 10μg/mL | |||
Human metapneumovirus(hMPV) | Human Metapneumovirus | 1.8mg/mL | 93.92% | 94.70% |
REF | MF-139 | |
Product Name | SARS-CoV-2/RhV/PIV/Influenza A/B / RSV/ADV/hMPV/M.P Antigen Combo Test Kit (Colloidal Gold Chromatographic Immunoassay) | |
Method | Colloidal Gold Chromatographic Immunoassay | |
Qualification | CE | |
Testing content | SARS-CoV-2(COVID-19) Rhinovirus(RhV) Parainfluenza viruses(PIV) Mycoplasma Pneumoniae(M.P) Influenza A(FLU A) Influenza B(FLU B) Respiratory syncytial virus (RSV) Adenovirus(ADV) Human metapneumovirus(hMPV) | |
Sample Type | Nasal swab, Oropharyngeal swab and Nasopharyngeal swab | |
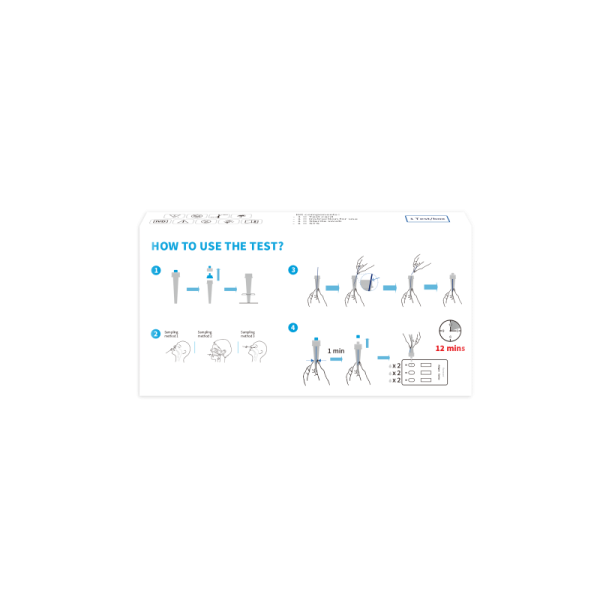
Reaction Time (min) | 12 mins | |
Storage Temperature | 2-30℃ | |
Shelf Life | 24 months | |
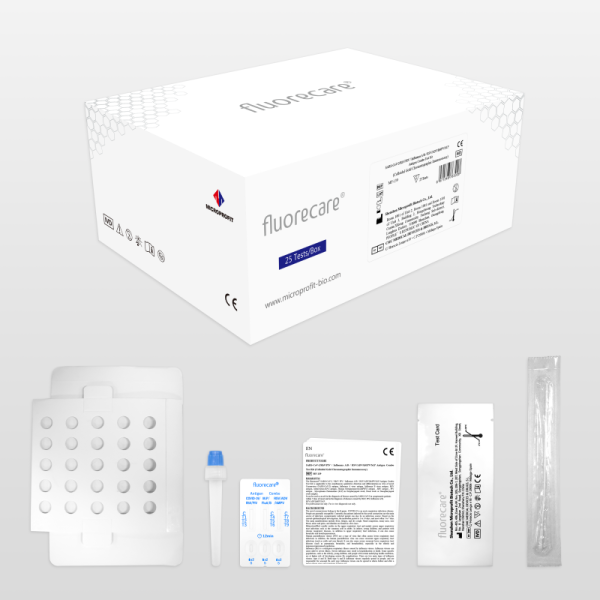
Specification | 1 tests/kit | 25 tests/kit |
Kit components | Instructions for Use:1 copy Sterile swab:1 piece STS(Sample treatment solution):1 tube | Instructions for Use:1 copy Sterile swab:25 piece STS(Sample treatment solution):25 tube |
Size(L*W*H)mm/Kit | 130*67*20 | 250*138*83 |
Weight(kg)/Kit | 0.03 | 0.49 |
Kits(t) | 500 | 40 |
Size | 67*35*43 | 58*44*43 |
Gross weight | 17.2 | 21.6 |
Net weight | 15.15 | 19.9 |




