Microprofit Biotech Shines at Medlab Middle East 2025
Medlab Middle East 2025 — the annual global event for the medical laboratory and in vitro diagnostics (IVD) industry — grandly opened at the Dubai World Trade Centre from February 3 to 6, 2025. This year's exhibition has attracted over 800 exhibitors and is expected to welcome more than 30,000 professional visitors to explore cutting-edge industry technologies and future development trends.

As an innovative enterprise in China’s IVD industry, Microprofit Biotech made a striking appearance at the event with a wide range of independently developed products. The showcased product lineup includes rapid tests for respiratory infections, monkeypox, and tropical diseases, FIA quantitative POCT platform, biological reagent raw materials, and intelligent diagnostic equipment — fully demonstrating the company’s technological innovation capabilities in the field of medical diagnostics.

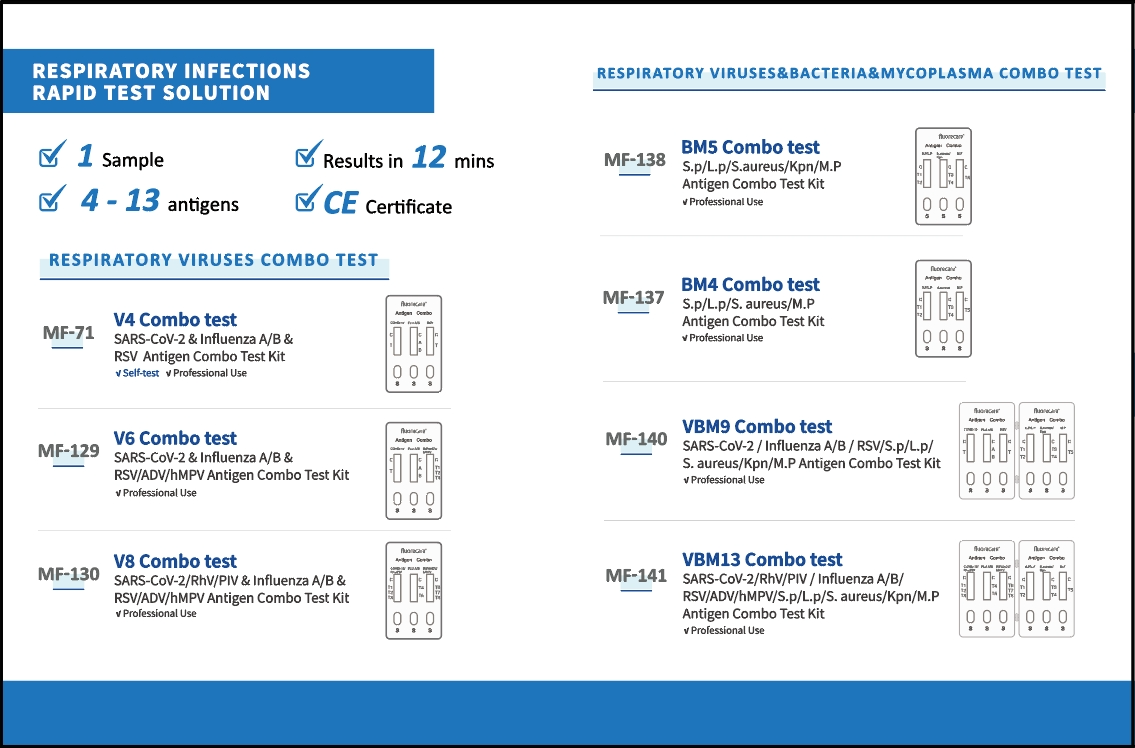
Amid the ongoing global influenza A outbreak, Microprofit Biotech’s self-developed multiplex in 1 respiratory pathogen detection reagent has attracted significant attention. Known for its high efficiency, accuracy, and reliability, the product allows for the rapid detection of 13 respiratory pathogens (including influenza A and B) from a single nasal swab sample within 12 minutes — anytime, anywhere, without the need for additional equipment or conditions. It covers over 98% of respiratory infection cases, detecting:
8 viruses (COVID-19, influenza A, influenza B, respiratory syncytial virus, adenovirus, rhinovirus, human metapneumovirus, parainfluenza virus)
4 bacteria (Streptococcus pneumoniae, Legionella pneumophila, Staphylococcus aureus, Klebsiella pneumoniae)
Mycoplasma pneumoniae
This comprehensive and efficient solution provides a powerful tool for the rapid diagnosis of respiratory infections.
Building on this core technology, Microprofit Biotech has developed seven combination products. Among them, the 4 IN 1 Respiratory Virus Combo Test Kit (for COVID-19, influenza A, influenza B, and RSV) has captured over 80% of the European market due to its accuracy, speed, and convenience, establishing itself as a benchmark product in the field of rapid respiratory infection detection. This series of innovative products not only provides efficient solutions for clinical diagnosis but also makes a significant contribution to global respiratory infection prevention and control.
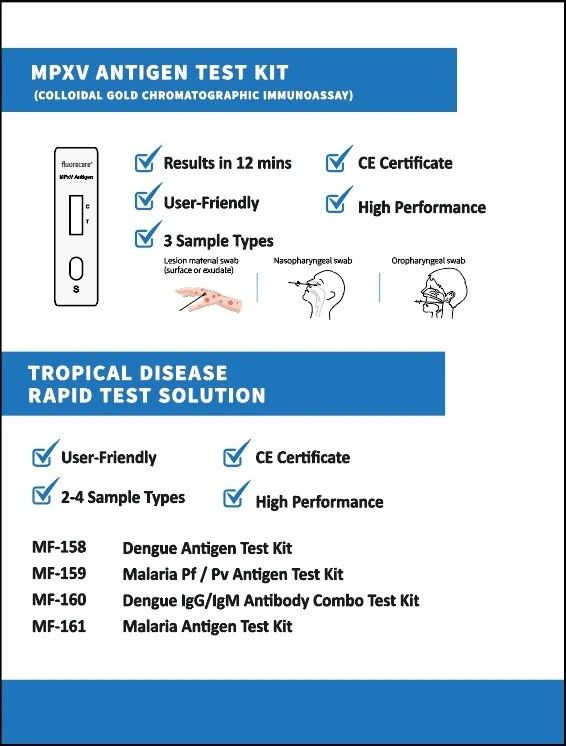
In response to the World Health Organization’s (WHO) recent warning about monkeypox virus infections, Microprofit Biotech introduced its self-developed Monkeypox Virus Rapid Test Kit and Tropical Disease Rapid Test Kit at Medlab 2025. These innovative products highlight the company’s advanced expertise in infectious disease diagnostics and its commitment to addressing global health challenges. This initiative reflects Microprofit Biotech’s global vision and social responsibility — offering strong technological support for global public health security.
In addition, Microprofit Biotech’s fluorecare® FIA Quantitative POCT Platform products and the latest integrated incubation immunofluorescence analyzer drew significant interest during the exhibition. With their ease of use, comprehensive parameter coverage, and accurate results, these products attracted numerous visitors eager to explore potential collaboration opportunities.
Microprofit Biotech’s rapid respiratory testing products hold a leading position globally, and its POCT platform stands out for its broad test coverage and superior performance — a testament to the company’s strong R&D capabilities.
To benefit global users and support the IVD industry’s development, Microprofit Biotech also showcased 24 high-quality biological reagent raw materials at the event. These materials, known for their outstanding performance and consistency, offer strong technical support for the next generation of IVD solutions. This display became one of the highlights of the exhibition, further underscoring Microprofit Biotech’s leadership in global IVD innovation.

As an innovative enterprise with over 15 years of experience in the IVD field, Microprofit Biotech has consistently adhered to independent innovation, continuously breaking technological barriers. To date, the company has obtained over 40 invention patents and more than 150 CE certifications, with products exported to over 100 countries and regions worldwide — earning broad recognition in the international market for its outstanding scientific research and product quality.
Participating in Medlab Middle East 2025 marks an important milestone in Microprofit Biotech’s global branding strategy and lays a solid foundation for expanding its international cooperation network. Looking ahead, Microprofit Biotech will continue to uphold the philosophy of "Innovation-Driven, Quality First," working hand in hand with global partners to drive medical technology innovation and contribute Chinese expertise to the advancement of global healthcare.

The exhibition is still in full swing! You are cordially invited to visit Booth Z2.F12 and explore the endless possibilities of medical technology with Microprofit Biotech as we shape a brighter future for the industry together!

